TELL ME MORE
ABOUT THIS CLINICAL TRIAL for ATOPIC DERMATITIS
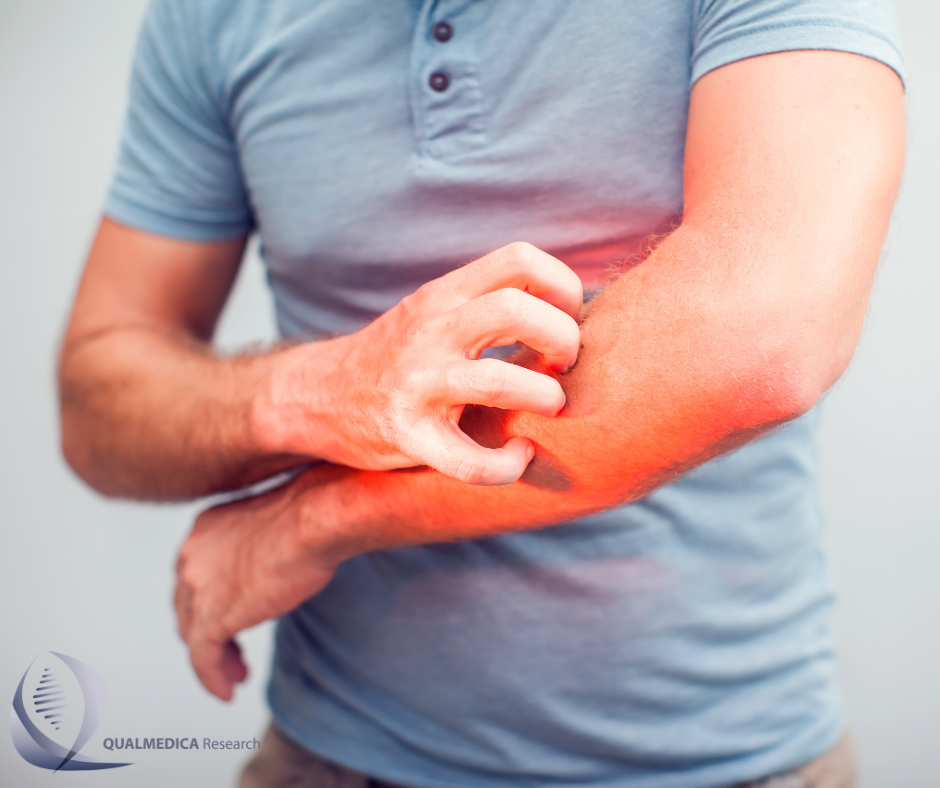
This is a Safety and Efficacy Study for children and adults two or more years of age with Moderate to Severe Atopic Dermatitis (Eczema)
Clinical Trial for Atopic Dermatitis
Study Location(s): Evansville Indiana & Bowling Green, KY
Atopic Dermatitis (AD) is a type of Eczema and is most frequently associated with dry, itchy scaly skin. The National Eczema Association estimates that over 30,000,000 people suffer with Eczema in the United States. More than half (56%) of all Eczema patients have Atopic Dermatitis. This study is an open-label, long-term extension trial.
There is no cost to participate. Volunteers receive free investigational medicine and follow-up care. No insurance needed. Time and travel reimbursement up to $975 may be available for participation.
